Behind the Biohazard Sign: Contextualizing Biosafety
In one of my earliest experiences working in a biological laboratory, a researcher jokingly referred to themselves as ‘a plumber, just for really small amounts of liquid.’ This lighthearted comment underscored the seemingly mundane yet meticulous nature of lab work which often involves hours, if not days, of moving extraordinarily small amounts of clear liquids around from one container to another, adding and taking away more clear liquids - hoping to uncover insights about the unseen world within those liquids. At the time I was told this joke, I was sitting in a BSL-2 laboratory, and if you had asked me to explain what BSL-2 meant, I could barely have told you that it stands for Biosafety Level-2, let alone detailed what that even means.
Now, I spend my days deeply immersed in biosafety regulations, learning the intricate rules (and sometimes lack thereof) that govern these spaces. These topics are often contentious, even among seasoned professionals, reflecting the complexity of the laboratory environments they aim to regulate. A true understanding of biosafety and its regulation requires a nuanced appreciation of the diverse laboratory settings where biological materials are handled. While it may be impossible to become an expert in every variation of biological research, this document endeavors to compile and describe these unique facilities around the world. My goal is that this serves as an in-between for those starting their journeys in infectious disease research or policy and that it helps the new biosafety professional or policy maker develop a strong fundamental understanding of biological research and introduce them to the resources needed to truly dive into this topic.
THE BASICS: describing biological research
The word ‘lab’ means something a little different to everyone. For some, it is where their GPA went to die in undergraduate chemistry, for others it is a place of innovation and tinkering, and for some it's very cute dogs. However, here, we are talking about laboratories that work with biohazardous materials or biological agents. These include the typical suspects like bacteria and viruses, but the definition of biohazardous materials is very broad and also includes things like prions, protozoa, fungi, biological toxins (often produced by bacteria), and even genetic material like recombinant DNA.
THE BASICS: risk groups
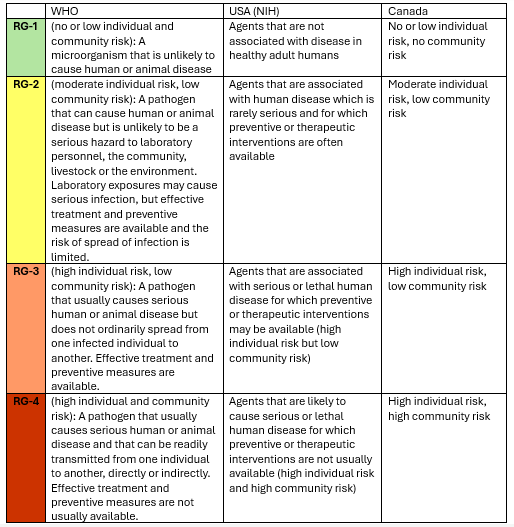
Around the world, biological agents are classified into risk groups. Risk Group (RG) classifications categorize biological agents based on their potential to cause disease in humans, animals, or plants and the likelihood of transmission. The Risk Group system ranges from Risk Group 1 (lowest risk) to Risk Group 4 (highest risk). Different countries may adopt slightly varying definitions and guidelines, but the core principles remain similar, and the classifications are recognized globally in many biosafety standards. Here, three frequently referenced RG classification definitions used around the world are shown:
Now, what does this mean in real life? Below is an example of the risk group classifications around the world for Bacillus anthracis, the causative agent of anthrax. This information is pulled from the ABSA risk group database.
Note how this agent is classified variably as RG-2 and RG-3 worldwide. This is an excellent example of how these definitions are flexible. When assigning agents to risk groups, many considerations are evaluated, such as pathogenicity, availability of treatments, host range, if a pathogen is foreign or endemic to that country, whether it is attenuated, etc. This can make regulation based on risk group very challenging as certain pathogens may have many different strains or other considerations that impact their virulence.
THE BASICS: biosafety levels
Risk group classifications are closely linked to specific containment procedures, personal protective equipment (PPE), and laboratory practices. These containment procedures are often structured into Biosafety Levels (BSL), which range from BSL-1 (lowest risk) to BSL-4 (highest risk). These safety levels vary in their requirements based on the organism or material being handled and depend on the local, national, and international regulations and guidelines that apply.
Now, as a biosafety professional, I have to stop us here to explain two gripes:
Biosafety levels and risk groups are not the same! While closely related, just because a bacterium is considered RG-2 does not mean it is only worked with in BSL-2. RG describes the pathogen, BSL describes the facility where work is allowed with that pathogen.
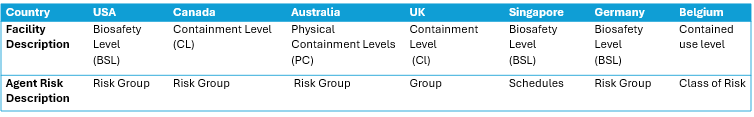
There is no universal terminology or definition for containment facility classifications or risk groups. I default to using the term BSL and RG because of my bias coming from the United States, but around the world different terminology and classifications are used.
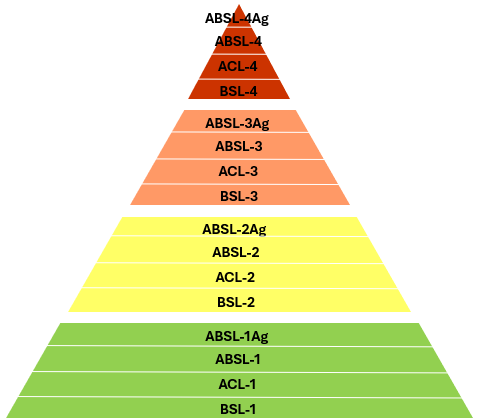
Regardless of what these facilities are called, or where they are located, they share many similar features. Here we will look at these levels in casual and high-level way, highlighting examples of these labs from around the world. Focus on the details of the pictures and exploring links to examples of laboratories at each level. Note that biosafety levels build upon each other, any items discussed at a lower level apply to all levels above.
BSL-1
BSL-1 facilities are typically teaching labs, or university research labs that work with non-hazardous materials such as plant pathogens. BSL-1 laboratories do not need to have access controls, and work is typically conducted on open benchtops using standard microbiological practices. PPE typically includes reusable lab coats, gloves, and safety glasses. Basic safety practices such as such as no eating or drinking and proper handwashing are followed.
BSL-2
BSL-2 facilities are commonly found in clinical, diagnostic, research, and manufacturing settings. Anecdotally this is probably the biggest category of labs around the world (it is very difficult to find data on lab numbers, especially for lower biosafety levels). These labs generally have limited access, specific training requirements, and require waste decontamination either on or off site. PPE may include lab coats, gloves, face protection, and sometimes respiratory protection. Biosafety cabinets (BSCs) are critical safety tools that are used for procedures that may create infectious aerosols or splashes. Biosafety cabinets use directional airflow and HEPA filtration to protect lab personnel, the environment, and samples from contamination. They draw air through front grilles, filter it, and maintain a sterile work area while preventing exposure to aerosols.
BSL-3
BSL-3 laboratories are primarily found at academic, government, and private institutions. Access to BSL-3 labs is strictly controlled and requires specialized training that typically involves mentoring and proficiency testing. Compared to BSL-1 and 2, these facilities have significantly more engineering controls, such as directional airflow and HEPA filtration. The labs are designed to be easily decontaminated from floor to ceiling and have waste decontamination systems built into the design. PPE could include a combination of scrubs, solid-front gowns, Tyvek suits, gloves, eye protection, and respirators. All work is conducted within biosafety cabinets to contain potential aerosols.
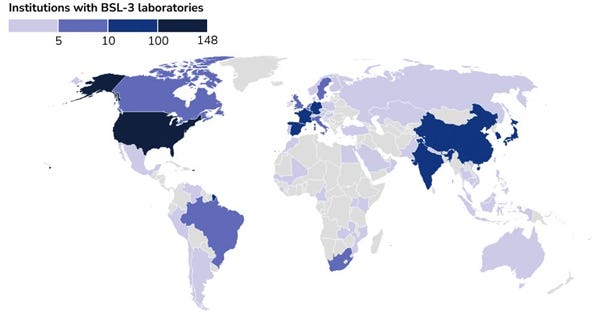
There is no comprehensive list of BSL-3 facilities, or the institutions in which they are housed, however the Georgetown Center for Emerging Technology has tried to map these facilities based on published literature. This is by no means a comprehensive list as labs that do not publish (such as diagnostic labs) or countries with less access to publishing will be underrepresented, however it helps give an idea of global scale.
BSL-4
BSL-4 labs are rare and are typically found in specialized research institutions funded by government programs. George Mason University hosts an interactive map that shows as of 2022 there are approximately 69 BSL-4 labs in operation, under construction, or planned in 23 different countries - quite an increase from the original 60 identified when the project began in 2021. Check out the map and a discussion of BSL-4 labs. These labs have the highest level of biocontainment, including sealed environments with controlled air filtration and waste management systems. There are two types of BSL-4 labs: suit labs and cabinet labs. In suit labs, personnel must wear fully encapsulating positive pressure air-supplied suits and work within the BSCs described at previous biosafety levels. Strict protocols for entry and exit including chemical showers for PPE ensure no contamination can leave the labs. Cabinet labs utilize class III biosafety cabinets, which are completely airtight isolated environments in which to perform work, this allows for less strict PPE as researchers never contact the agents directly. As you can imagine each of these setups have its unique strengths and weaknesses, most BSL-4 work occurs in suit labs.
BSL-5: Alien Biosafety
…just kidding. There is no BSL-5, but there is serious working being done by NASA and ESA to potentially bring back the first Martian samples to Earth in the early 2030s. In preparation a Mars Sample Receiving Facility (SRF) is being planned which ‘would feature biosafety level 4 (BSL-4)-like high containment to protect Earth’s biosphere from any potential hazard’.
→ NASA Report | The New York Times Article ← if you’d like to learn more
Now, back to business…
To summarize and immerse yourself in biosafety levels I strongly encourage you to watch this brief Texas Biomedical Research Institute virtual lab tour. If you want an in-depth, technical breakdown of biosafety levels, I recommend reviewing your country's specific regulations.
Note this is not an exhaustive list, but an overview of the main biosafety documents / regulations in countries with strong biosafety programs. Later in this post we will use the United States as an example of how complex these documents can be.
This still leaves a big question- what about countries that do not have their own biosafety guidance and regulation? In these instances, laboratories may adopt the practices outlined by other counties, often the United States Biosafety in Microbiological and Biomedical Laboratories (BMBL) or the World Health Organization (WHO) Laboratory Biosafety Manual (LBM). The WHO LBM attempts to acknowledge both the extraordinary variety of research performed and the discrepancy of resources around the world. Instead of identifying laboratory levels, the WHO LBM has transitioned from defining biosafety levels to a risk-based approach that broadly defines labs by the measures needed to perform work safely. These risk- based categories are: core requirements, heightened control measures, and maximum containment measures. They can be loosely translated to biosafety levels as follows.
BEYOND THE BASICS: capturing different types of research within biosafety levels
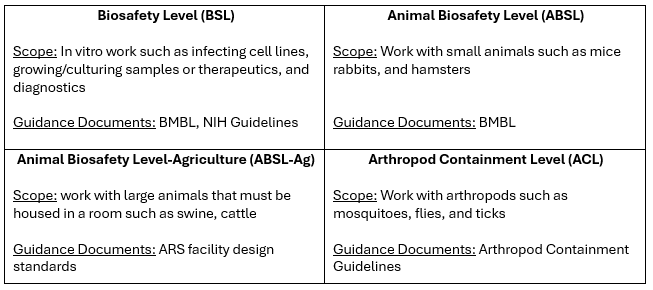
Broadly defining biosafety levels is not enough to fully capture the breadth of work that is done in laboratories that work with biohazardous materials. To get an idea of the complexity of this here is a look at the types of biosafety levels in the United States, the worked performed in each type and where you can find guidance documents.
I specifically use the term ‘guidance document’ here because none of these documents are regulatory with legal weight. In fact, the BMBL, often referred to as the biosafety bible, specifically prefaces that it is an advisory document and ‘not intended to be a regulatory document, although we recognize that some may use it in that way’. The NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules is an example of a weird gray area that exists in much of the United States research regulation; its regulatory reach is tied to the funding source of the work. Research that is federally funded must follow the NIH Guidelines (for example universities receiving NIH grants) but is otherwise non-binding (for example private for-profit organizations that are internally funded).
This is not to say however that there are no regulations, in fact there are many…
Biological Transport Regulations (49 CFR 173.132–134),
CDC Import Permits (Public Health Service Act, 42 U.S.C. § 264),
Environmental Impact Assessments for Biological Materials (National Environmental Policy Act (NEPA) Regulations, 40 CFR Parts 1500-1508),
OSHA Bloodborne Pathogens Standard (29 CFR 1910.1030),
Animal Welfare Act (7 U.S.C. 2131-2159),
Public Health Service Policy on Humane Care and Use of Laboratory Animals (42 CFR Part 93),
Select Agent Regulation (7 C.F.R. Part 331), USDA Animal and Plant Import Permits.
All of these regulations have different regulatory authorities and requirements for who must follow them based on research type, funding, and biological materials being used. And that’s not to mention that state and local laws that laboratories must follow which is where most regulated medical waste disposal regulations are captured. It’s complex, as it must be to reflect the complex diversity of research. But the question I won’t try to answer here is whether complex means it must be complicated?
If you put it all together the diagram of biosafety programs in the United States (and many other countries) might look something more like this:
However, knowing the regulatory background and classification of laboratories is really just the beginning of understanding work with biohazardous materials. To truly understand the complexity of these labs you have to consider the huge variety of work performed. There are a few major categories of labs. One of the first big splits is diagnostic labs versus research and development. Diagnostic labs could be state of the art public health labs, or they could be mobile field labs responding to emerging outbreaks. Research labs include universities and private companies as well as government facilities that could be doing anything from protein isolation to pathogen sequencing to drug discovery. Then there are commercial labs working on drug pipelines from early discovery to clinical trials. There are labs that focus on plant pathogens or work with animals. Labs that research viruses in insects. Pathology labs, microscopy cores, genetic engineering, synthetic biology, wastewater analysis. The list goes on.
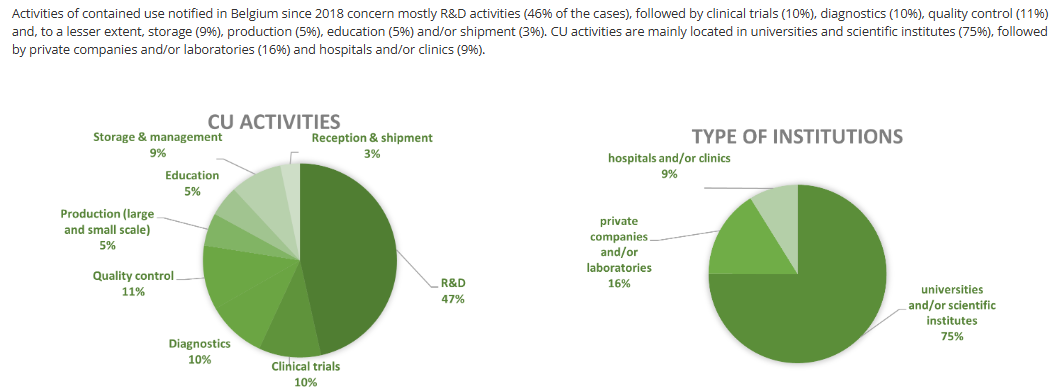
Every country is going to look different, but the Belgium Biosafety Server has some interesting data on the types of activities and facilities that work with genetically modified and/or pathogenic organisms (contained use, or CU, per their terminology) to help give a better idea of the landscape of biological research.
While numbers are great, the best way to understand the breadth of research and corresponding biosafety considerations is to see it. Take some time to explore the following video links to get an idea of what lab work looks like around the world in different facilities. At minimum I recommend watching the Texas Biomed tour linked earlier. If you only have 10 minutes more to explore, I recommend watching videos 2, 4, and 7 in entirety and then a few minutes of 6. If you have 15-20 minutes skip around through the rest of the videos based on your interests.
1. Working in ABSL-1 (10 minutes)2. Working in the BSL-4: Positive Pressure Suits (1 minute)3. Tour of the European Mobile Laboratory in Foya, Liberia (7 minutes)4. A Day in a Life of a Medical Laboratory Technician (4 minutes)5. Handling Sheep and Goats at an ABSL-3Ag Facility (7 minutes)6. Passaging Cells: Cell Culture Basics (5 minutes)7. Developing Biological Drugs (4 minutes)A PARTING NOTE
The work conducted in biological laboratories spans a wide spectrum of activities, each requiring specialized knowledge, expertise, and containment strategies. Whether in diagnostic labs responding to outbreaks, research labs exploring fundamental biological mechanisms, or commercial labs advancing new drug discoveries, these laboratories play a critical role in protecting public health, advancing science, and developing new medical technologies. The diversity in the types of work and the organisms studied reflects the complexity of modern biosafety and the need for specialized containment strategies tailored to each unique research goal.
I hope this helped introduce biosafety and left you with questions to explore and the resources to start your explorations. And lastly, for both those newly introduced and those in the trenches, I hope it helps remind us that biosafety is not mean to be siloed. We need to reach outside of our biosafety cabinets, our labs, and into other industries and other countries to build biosafety practices and policies that are both as unique as the individual work it supports, and as connected as the world it safeguards.
ADDITIONAL RESOURCES
Cao, Cong. “China's evolving biosafety/biosecurity legislations.” Journal of law and the biosciences vol. 8,1 lsab020. 30 Jun. 2021, doi:10.1093/jlb/lsab020
Gillum, David R et al. “Establishing a national biosafety and biosecurity agency for the United States.” Frontiers in bioengineering and biotechnology vol. 12 1474120. 17 Oct. 2024, doi:10.3389/fbioe.2024.1474120
Johnson, Barbara, and Rocco Casagrande. “Comparison of International Guidance for Biosafety Regarding Work Conducted at Biosafety Level 3 (BSL-3) and Gain-of-Function (GOF) Experiments.” Applied biosafety : journal of the American Biological Safety Association vol. 21,3 (2016): 128-141. doi:10.1177/1535676016661772
Schuerger, Caroline, Sara Abdulla, and Anna Puglisi. "Mapping biosafety level-3 laboratories by publications." Center for Security and Emerging Technology. https://doi. org/10.51593/20220019 (2022).